Chemical Sensors: Design, Operation, Types and Comparison with Biosensors
Catalog
What Is a Chemical Sensor?Structure of a Chemical SensorWorking Principle of a Chemical SensorChemical Sensor Circuit and How It WorksCircuit Operation: Potentiostat and Signal ConversionTypes of Chemical SensorsDifference Between Chemical Sensors and BiosensorsAdvantages and Disadvantages of Chemical SensorsApplications of Chemical SensorsFrequently Ask QuestionsRelated ArticlesAn ideal chemical sensor is a compact, cost-effective, and user-friendly device that offers immediate and highly selective responses to a specific target substance (analyte) in any chosen medium, producing a clear and quantifiable signal at any desired concentration level. Typically, these sensors are tools or instruments used to determine the amount, presence, or concentration of a chemical substance.
The complexity of applying chemical sensors often depends on the technical challenges involved in detecting the substance and the specific characteristics of the material being analyzed. Factors such as the sensor’s selectivity and sensitivity can be influenced by dimensions, phase states, and timing aspects of the measurement process. Analytes may exist in liquid or solid form and can vary widely in quantity—from large volumes to extremely small scales like picoliters.
This article gives an overview of how chemical sensors work, their key components, the various types available, and their real-world applications.
What Is a Chemical Sensor?
A chemical sensor is a device designed to identify and measure specific chemical properties within a sample—such as its composition, the presence of a particular element or ion, chemical activity, or concentration—and convert that information into an electronic signal. These sensors are widely used in various fields, including home safety systems, medical diagnostics, nanotechnology, and the automotive industry.
Structure of a Chemical Sensor
The basic structure of a chemical sensor consists of two main parts: a sensing element (receptor) and a transducer. The sensing material interacts with the target analyte in a way that depends on the sensor’s type. This interaction causes a change in a measurable property of the material—such as electrical conductivity or mass—which the transducer then converts into a readable electrical signal.
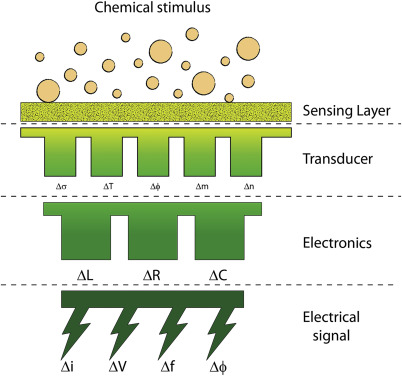
Chemical Sensors
Transducer Function in a Chemical Sensor
The transducer is the second key component of a chemical sensor. Its role is to convert the chemical interaction between the sensing element (receptor) and the analyte into an electrical signal. This signal is then sent to a computer or a mechanical system for further processing, monitoring, or control.
Working Principle of a Chemical Sensor
Chemical sensors operate based on electrochemical reactions, where the presence, composition, or concentration of organic or inorganic compounds is detected and converted into an electrical signal. This allows for precise measurement and real-time monitoring of various chemical substances.
Chemical Sensor Circuit and How It Works
A typical example of a chemical sensor circuit is a carbon monoxide (CO) sensor. This sensor includes three electrodes—a working electrode, a counter electrode, and a reference electrode—all immersed in a liquid electrolyte. Among them, the working electrode is the most critical and is usually made of platinum, a catalytic metal ideal for carbon monoxide detection.
The working electrode is supported by a gas-permeable, hydrophobic membrane, which allows CO gas to pass through while keeping the liquid out. Once the carbon monoxide molecules diffuse through the membrane, they reach the electrode surface and undergo electrochemical oxidation. This reaction generates an electrical signal that corresponds to the concentration of CO in the environment.
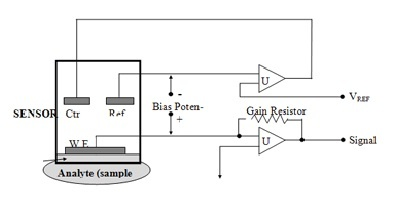
Carbon Monoxide Chemical Sensor Circuit
Signal Generation and Electrochemical Roles of Electrodes
The output signal from the chemical sensor is generated by the electrons produced during the electrochemical reaction at the working electrode.
The reference electrode plays a crucial role by providing a stable electrochemical potential within the electrolyte. It is isolated from exposure to carbon monoxide gas, ensuring that its thermodynamic potential remains constant. Importantly, no current flows through the reference electrode, allowing it to act purely as a voltage reference.
The counter electrode completes the electrochemical cell. It functions as the second half-cell, allowing electrons to either enter or exit the electrolyte as needed to maintain the reaction balance.
Circuit Operation: Potentiostat and Signal Conversion
The circuit controlling this sensor includes a potentiostat, which maintains a fixed potential at the working electrode (WE) and converts the resulting sensor current into a voltage output. The U2 operational amplifier (op-amp) performs this current-to-voltage conversion.
At the same time, the reference electrode (RE) voltage is compared against a fixed input voltage called Vbias. The U1 op-amp in the circuit adjusts the counter electrode (CE) voltage to ensure the current flowing from CE is equal in magnitude and opposite in direction to the current from WE. This setup ensures a stable voltage is maintained between the working and reference electrodes, which is essential for accurate sensing.
Selective Filtering and Sensor Adaptability
To enhance selectivity, the carbon monoxide sensor includes a chemically selective filter. This filter blocks interfering gases from reaching the working electrode, ensuring that only the target gas (CO) triggers a response. If the filter operates effectively, the sensor will show minimal response to unwanted gases, improving accuracy.
This sensing technology can be adapted to detect various gases. By modifying the working electrode material, filter type, and bias voltage, different sensors can be developed for different target gases.
Types of Chemical Sensors
There are several types of chemical sensors, each designed for specific applications. Below is an overview of one commonly known example:
Breathalyzer
A breathalyzer is a chemical sensor used to estimate Blood Alcohol Content (BAC) by analyzing a person's breath. When someone consumes alcohol, a portion of it is exhaled in their breath in proportion to the amount they’ve consumed. The breathalyzer is specially designed to measure BAC levels and is often used to assess whether an individual is fit to drive safely.
When alcohol molecules in the breath reach the sensor’s receptor, they react with a chemical solution inside the device. This solution typically contains substances like potassium dichromate, sulfuric acid, silver nitrate, and water. The interaction between the alcohol and these chemicals causes a change in chemical balance between two chambers. This change triggers the generation of an electrical signal, which is then displayed on a needle gauge or digital screen to show the BAC level.

Breathalyzer Sensor
Carbon Dioxide Sensor
A carbon dioxide sensor, commonly referred to as a CO₂ sensor, is a device used to detect and measure the concentration of carbon dioxide gas in the environment. These sensors typically operate based on two main principles: infrared (IR) absorption and chemical reaction.
Measuring CO₂ levels is essential in a wide range of applications, such as:
- Monitoring indoor air quality in homes, offices, and public buildings
- Medical devices like capnographs, which evaluate lung function by measuring CO₂ in exhaled breath
- Industrial processes where maintaining proper CO₂ levels is critical for safety or efficiency
CO₂ sensors play a key role in ensuring healthy environments, optimizing ventilation systems, and supporting accurate medical diagnostics.

Carbon Dioxide Sensor
Carbon Monoxide Detector
A carbon monoxide detector is a safety device designed to detect the presence of carbon monoxide (CO) gas and help prevent CO poisoning. Carbon monoxide is a colorless, odorless, and tasteless gas that is produced when fuels containing carbon are only partially burned—for example, in malfunctioning heaters, stoves, or engines.
Because CO is difficult to detect without equipment, it poses a serious health risk. High concentrations or prolonged exposure can be extremely dangerous—even fatal.
Carbon monoxide detectors are engineered to continuously monitor the environment for rising CO levels. If the concentration reaches a dangerous threshold, the device triggers an alarm, giving people enough time to ventilate the area or evacuate safely.
Electronic Nose (E-Nose)
An electronic nose, or e-nose, is a device designed to detect and identify odors or flavors, mimicking the human sense of smell. It uses a sensor array combined with pattern recognition algorithms to analyze complex chemical mixtures in the air.
The operation of an electronic nose closely mirrors the process of human olfaction. It typically involves several stages such as:
- Identification – recognizing a specific odor signature
- Comparison – evaluating similarities or differences between smells
- Quantification – measuring the intensity or concentration of detected compounds
- Data Storage and Retrieval – saving odor profiles for future analysis or reference
E-noses are used in various fields, including food and beverage quality control, environmental monitoring, medical diagnostics, and security systems.

Electronic Nose
Zinc Oxide Nanorod Sensor (ZnO Nanorod Sensor)
A zinc oxide nanorod sensor is a type of optical or electronic chemical sensor designed to detect the presence of specific gas molecules or liquids in the surrounding environment. It leverages the unique properties of ZnO nanorods, which have a high surface-to-volume ratio, making them highly sensitive to molecular interactions.
When molecules are absorbed onto the surface of these nanorods, they cause measurable changes in certain properties such as:
- Photoluminescence
- Vibration frequency
- Electrical conductivity
- Mass
The most widely used and straightforward detection method involves passing an electrical current through the ZnO nanorods and monitoring any change in conductivity when exposed to target gases. This makes ZnO nanorod sensors ideal for applications requiring high sensitivity and fast response times.

Zinc Oxide Nanorod
Potentiometric Sensor
A potentiometric sensor is a type of chemical sensor used to determine the concentration of specific components within a gas or liquid sample. It works by measuring the electrical potential (voltage) of an electrode under conditions where no current is flowing.
These sensors are known for their simplicity, low cost, and ease of use, making them a practical alternative to more complex analytical instruments. Due to their versatility, potentiometric sensors are widely used across various sectors, including:
- Food safety and quality monitoring
- Healthcare and medical diagnostics
- Agriculture
- Water and environmental quality monitoring
- Personal health monitoring systems
Their ability to provide quick, on-site measurements makes them especially valuable for real-time analysis in field or clinical settings.

Potentiometric Type
Hydrogen Sensor
A hydrogen sensor is a device designed to detect the presence of hydrogen gas in various environments. These sensors are valued for being affordable, durable, compact, and easy to maintain compared to many other types of gas sensors.
Since hydrogen is a colorless, odorless, and tasteless gas, it cannot be detected by human senses, making these sensors essential for monitoring hydrogen levels and detecting gas leaks. Hydrogen sensors are commonly used in safety systems, industrial settings, and hydrogen-powered technologies to provide early warnings and prevent hazardous situations.

Hydrogen Sensor
Fluorescent Chloride Sensor
A fluorescent chloride sensor is a specialized chemical sensor used to measure chloride ion (Cl⁻) transport across cell membranes. This transport plays a vital role in regulating cell volume, electrical charge balance, membrane excitability, and the resting potential of cells.
These sensors are especially important in medical diagnostics, notably in detecting cystic fibrosis, a condition linked to abnormal chloride transport. Advances in understanding chloride’s role in physiological processes have driven the development of fluorescent tools that allow real-time measurement of intracellular chloride levels in living cells.

Fluorescent Chloride Sensor
Difference Between Chemical Sensors and Biosensors
| Chemical Sensor | Biosensor |
|---|---|
| A chemical sensor is an analytical device that converts a chemical signal into an electrical signal. | A biosensor is an analytical device that detects chemical substances by integrating a biological component with a physicochemical detector. |
| It uses a receptor and a transducer to sense chemical compounds. | It combines biological elements (like enzymes, antibodies, or cells) with physical or chemical transducers. |
| Primarily measures and characterizes chemical compounds. | Primarily measures and characterizes organic or biological materials. |
| Common examples include breathalyzers, electrochemical gas sensors, and carbon monoxide detectors. | Common examples include pregnancy tests and glucose monitoring sensors. |
| Widely applied in fields such as environmental monitoring, food safety, mining, medical diagnostics, defense, and bioengineering. | Used for disease monitoring, pollutant detection, drug development, and identifying disease-causing microorganisms. |
Advantages and Disadvantages of Chemical Sensors
Advantages:
- Provide fast and reliable responses to a wide range of gases and vapors.
- Generally affordable and cost-effective.
- Simple to operate and highly portable, making them suitable for field use.
- Typically low maintenance and inexpensive to replace.
Disadvantages:
- Often have a limited temperature range in which they can operate effectively.
- May not fully satisfy all environmental monitoring requirements due to sensitivity or selectivity constraints.
- Usually have a limited shelf life, which can affect long-term usability and accuracy.
Applications of Chemical Sensors
Chemical sensors play a crucial role in many fields, including:
- Medical diagnostics: Monitoring biochemical markers for health and disease detection.
- Environmental monitoring: Detecting pollutants and contaminants in air, water, and soil.
- Food industry: Ensuring product quality and safety through chemical analysis.
- Bioengineering and biotechnology: Supporting research and development processes.
- Defense and safety: Detecting hazardous gases and ensuring workplace safety.
- Mining and industrial processes: Controlling product quality and process conditions.
These sensors are widely used in applications such as safety monitoring, critical care, industrial hygiene, process control, and quality assurance.
Chemical sensors help measure and detect chemical properties in various analytes and are also integrated into devices for home security and pollution detection.
In broader terms, chemical sensing technology supports a variety of disciplines like electrochemical analysis, biomedical measurements, pollution control, and industrial automation.
For more detailed examples and interfacing, consider looking into sensors such as:
- MQ-4 Methane Gas Sensor
- MQ-8 Hydrogen Gas Sensor
In summary, this overview covered chemical sensors—their structure, working principles, circuits, types, key differences from biosensors, advantages, disadvantages, and applications. Chemical sensors are devices that convert chemical signals into measurable analytical signals. These chemical signals arise from selective interactions between the sensor’s sensing material and a specific target analyte. Examples of chemical sensors include carbon monoxide detectors, glucose sensors, mosquito sensors, and pregnancy tests.
Now, here’s a question for you: What is a biosensor?
Frequently Ask Questions
Examples of Chemical Sensors
Chemical sensors are devices that measure and characterize chemical compounds. In contrast, biosensors focus on detecting and analyzing organic materials.
- Examples of chemical sensors: Breathalyzers, electrochemical gas sensors, carbon monoxide detectors.
- Examples of biosensors: Pregnancy tests, glucose monitoring sensors.
What Device Detects Chemicals?
An electrochemical sensor detects and measures chemical substances by sensing changes caused by the interaction between the chemical agent and the electrical properties of a circuit (Taylor and Schultz, 1996).
Advantages of Chemical Sensors
Solid-state chemical sensors offer several advantages over traditional analytical methods:
- High sensitivity and selectivity
- Excellent reliability
- Ability for real-time, in situ monitoring These features make them highly effective for continuous monitoring applications.
Difference Between Physical and Chemical Sensors
- Physical sensors measure physical quantities such as pressure, force, acoustics, flow, temperature, light, or magnetic fields.
- Chemical sensors detect chemical substances including gases, ions, odors, and biological molecules.
How to Detect Toxic Chemicals?
Toxic chemicals are commonly detected by analyzing biological samples like blood and urine, but sometimes hair or fingernail clippings are also used for heavy metal and toxin testing.
Can Sensors Detect Chemicals?
Yes, chemical sensors are widely used to detect toxic chemicals, gases, and biomolecules. Their applications include:
- Detecting contaminants like petroleum or mercury in soil
- Monitoring air quality and hazardous gases
- Identifying deadly chemical warfare agents, such as nerve agents
Related Articles
Preamplifiers: What They Are, How They Work, Types and Differences
Metal Oxide Film Resistors: Structure, Operation and Key Specs
Semiconductor Fuse: Structure, HSN Code, Operation, and Common Uses
DC Servo Motor: Structure, Operation, Arduino Interface & Common Uses
Iron Core Inductor : Construction, Formula, Working & Its Applications
Air Core Inductors: Design, Operation, Inductance, and Common Uses
Logic Analyzers: Overview, Function, Types, Comparison & Maintenance
Passive High-Pass Filter: Overview, Circuit Design, Operation & Types
Subscribe to JMBom Electronics !